Robert Jay Rowen*, Sharon Grabovac, Teresa B. Su
Rowen-Su Medical Clinic, Santa Rosa, CA, USA
*Correspondence to: Robert Jay Rowen, MD, drrowen@att.net.
orcid: 0000-0002-5717-5720 (Robert Jay Rowen
Abstract
Therapeutic use of ozone is becoming increasingly prevalent worldwide. New methods of administration are emerging. One of these emerging techniques, which we refer to as ozone dialysis, uses a dialysis membrane that allows blood to flow against a countercurrent of ozone gas. We found that ozone uptake by continuous countercurrent blood flow is at least 3 times higher than any comparable form of blood ozone administration currently available. This is the first quantitative report of ozone uptake by blood using the ozone dialysis technique.
Key words: direct intravenous ozone delivery; extracorporeal blood oxygenation and ozonation; hyperbaric ozone therapy; major autohemotherapy; ozone dialysis; ozone high-dose therapy; ozone therapy; recirculatory hemoperfusion
doi: 10.4103/2045-9912.356474
How to cite this article: Rowen RJ, Grabovac S, Su TB. Ozone dialysis delivers three or more times the ozone than other forms of ozone
blood treatment. Med Gas Res. 2023;13(2):67-71.
INTRODUCTION
Ozone therapy involves the use of an oxygen/ozone (ozone) gas mixture for treatment. Conventional ozone therapy utilizes a variety of techniques to deliver ozone to the blood. These techniques include direct intravenous ozone injection (DIV),1 major autohemotherapy (MAH),²⁻⁴ and hyperbaric ozone therapy (HBO₃ ). Typically, the DIV procedure involves introduction of a 27-gauge butterfly needle and slow instillation of an ozone gas mixture instilled into the vein. Volume usually begins at 20 mL of gas and concentration usually begins at 30 μg/mL ozone. Maximum volume ranges from 60 mL to 120 mL of gas. MAH consists of withdrawing a specified amount of blood (usually 200 mL) into a plastic bag and the addition of an equal volume of ozone gas (general concentrations of 20–50 μg/mL). The gas and blood phases are gently mixed and then the ozonated blood returns to the patient. In HBO₃ , 200 mL of blood is withdrawn under vacuum into a glass container, 200 mL ozone gas mixture at a concentration of 70 μg/mL is added under pressure up to 2 ATA (hyperbaric, 1 ATA = 760 mmHg), and then the treated blood, still under pressure within the glass bottle, is returned to the patient. (The treatment and return of 200 mL of blood with ozone is called one “pass” HBO₃ ).
Maximum theoretical delivery of ozone in these methods with the above parameters would be 4800 μg for DIV (maximum volume of 120 mL and gas concentration of 40 μg/mL), 10,000 μg for MAH, and 14,000 μg for a single 200 mL “pass” of HBO₃ . Lahodny⁵ has pioneered “multipass” delivery using HBO₃ . Therein, the pressurized 200 mL “hyperbaric” procedure is repeated 9 more times in a single sitting for a total of 10 “passes” of HBO₃ netting a total of up to 140,000 μg of ozone delivered. This has been termed “ozone high-dose therapy” (OHT) by Lahodny⁵ and has become popular worldwide. He reported extraordinary rapid healing of wounds with this method. It is commonly referred to as “10 pass” amongst American users. König and Lahodny⁶ have recently shown that OHT stimulates mitochondria in blood mononuclear cells.
Ozone therapy can also be administered to the body for local and systemic treatment by rectal insufflation of gas. Dosing, depending on condition treated, ranges from 10–300 mL oxygen/ozone gas mixture at 10–70 μg/mL.³,⁷,⁸
Ozone is known to have a hormetic effect, inducing positive effects on physiology even at low doses.⁹ Little is reported on the efficacy of higher doses of ozone vs. lower dose. However, some studies10-13 have discussed the results of OHT as pioneered by Lahodny. OHT safety was reviewed and reported at the annual meeting of the American Academy of Ozonotherapy.¹⁴ Based on a survey of OHT therapists, in over 4000 high-dose treatments, no significant toxicity or untoward effects were observed, except for transient “rust” colored urine in a small minority of patients.¹⁴ Survey participants reported uniform higher clinical efficacy over their previously used low doses. The purpose of the survey was to gather data on possible untoward effects of ozone at doses higher than those commonly used.
In the last two decades, a new form of ozone blood delivery has been developed, commonly referred to as extracorporeal blood oxygenation and ozonation (EBOO)¹⁵,¹⁶ or recirculatory hemoperfusion (RHP).¹⁷ A simpler more descriptive term to describe this countercurrent administration of ozone gas using semi-permeable dialysis membranes is “ozone dialysis.” The actual “ozone” gas used is a mixture of oxygen and ozone with a concentration of ozone up to 50 μg/mL gas for dialysis, but typically, 30 μg/mL is the maximum concentration currently used.¹⁸
Di Paolo et al.¹⁹ reported on “EBOO” oxidation effects in 2005. They found that after a session of EBOO, the interaction of ozone with blood components resulted in 4–5-fold increased levels of thiobarbituric acid reactants and a proportional decrease in plasma protein thiols without any appreciable erythrocyte hemolysis. Furthermore, in animals, the authors found that EBOO is “completely atoxic” over a wide time period and a wide dose range (20–60 μg/mL, with no clinical side effects during or after treatment.²⁰ In humans, EBOO clearly has clinical efficacy, which is superior to prostacyclin alone in the treatment of peripheral arterial disease19 and necrotizing fasciitis.²¹ These human studies used a very low concentration of ozone at 0.5–1.0 μg/mL. Unfortunately, all these EBOO studies reported gas flow as a pressure but not volume. Hence, there is no way to know how much ozone gas is provided for uptake, and there is no confirmation of analysis of gas concentration at either end of the apparatus. At such a very low concentration of ozone gas reported in the human studies, it would be impossible to accurately determine ozone uptake.
In EBOO/ozone dialysis/RHP methods, a peristaltic pump is utilized to extract blood from a peripheral vein into the dialysis chamber, where blood flows around tiny tubules in the chamber while ozone gas flows in the opposite direction (as a countercurrent) inside the tiny tubules in the dialysis chamber. The ozonated blood then returns by the peristaltic pump to the patient through a second peripheral vein. In contrast to the other forms of ozone delivery, little is known about actual ozone uptake by this method. For example, in routine hyperbaric ozone therapy, knowing the instant reaction of ozone and blood, it is accepted that 14,000 μg is delivered in a 200 mL “single pass” treatment. Therefore, if this is done a total of 10 times in one sitting, 140,000 μg of ozone would be delivered.
With DIV, it can be reasonably assumed that all the ozone in the syringe can be included in “uptake” as it is administered directly into blood. With gravity MAH, the uptake is a bit cloudier. Assuming 100% of the ozone added to the bag reacts with blood and not plastic (the bag), then giving 200 mL of blood treated with 200 mL of ozone gas at 50–70 μg/mL would provide 10,000–14,000 μg in the treatment. However, this method involves gently agitating the blood and gas for several minutes, during which some of ozone could be neutralized by the plastic bag itself.
We undertook this study solely to quantify the amount of ozone uptake in μg in patients receiving ozone dialysis during their routine treatment. Ozone dialysis methods have been performed for decades, at least 22 years in Malaysia alone, with no significant toxicity observed in over 200,000 treatments.22 There are no published or reported toxicity or untoward effects. We report our measurements of ozone uptake with the dialysis method and compare it to the current standard of OHT, the 10- Pass. We did not undertake this study to report on the clinical benefits or risks of ozone therapy based on dose.
PARTICIPANTS AND METHODS
Informed consent was obtained from regular medical clinic patients for their routine ozone therapy. Inclusion criteria were solely consecutive patients undergoing dialysis as their routine prescribed therapy. The measurements in no way affected the delivery or modification of their treatments. There were no exclusion criteria.
Ozone gas was generated by a Zotzmann Ozone 2000 device (Zotzmann and Stahl GmbH KG, Berglin, Germany). Ozone gas was delivered at 10–60 μg/mL, with most measurements conducted when delivered ozone gas concentration was 30–40 μg/mL and at a gas flow rate of 0.9 L/min.
Patients received one intravenous catheter in each arm, 20-gauge each, one for blood outflow and one for blood return. Blood was circulated through a Renak CTA (cellulose triacetate) 1500 dialysis chamber (SB-KAWASUMI, Sapporo, Japan) by a Harvard P1500 peristaltic pump (Harvard Apparatus, Holliston, MA, USA) through 7 mm inner diameter silicone tubing. The system was heparinized with a slow intravenous drip of 1 L of normal saline containing 15,000 units of heparin, of which only about 300 mL of the heparin solution actually goes into the patient during the course of the 1-hour treatment. (An additional amount of the heparin solution was used for priming and flushing the lines before and after the hour treatment).
Fresh ozone concentration was measured at the outlet of the Zotzmann ozone generator machine, and spent gas was analyzed for residual ozone at the exit of the dialysis system, just before the spent gas went through an ozone destruct device which converts ozone back to oxygen before the exhaust goes into room air. The patient’s routine treatments were not affected by any measurement. An IKO ozone analyzer (Zotzmann and Stahl GmbH KG) was used for all measurements, and to confirm generator output. We took measurements at 15-minute intervals to determine if various ozone concentrations would have a significant effect on blood ozone absorption (uptake).
To determine whether the selected concentration of ozone remained consistent/stable after flow through the dialyzer and tubing, measurements were also taken of the ozone gas after flow through a dry dialyzer at each concentration and compared to the corresponding selected ozone concentration. We found an average loss of 23% measured after 15 minutes at each concentration, and the loss was consistent with multiple measurements. We found the same loss if normal saline was loaded into the dialyzer. In our data below, we subtract the measured loss (in μg/mL) for each concentration to correct for non-blood absorption.
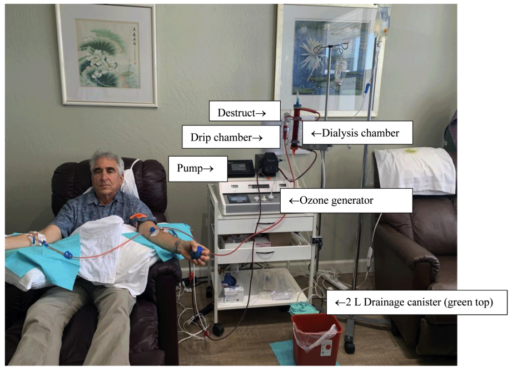
For the patients receiving their routine treatment, ozone was generated and delivered at varying concentrations during which measurements were taken. Specifically, measurements were taken 15 minutes after a change in ozone concentration or blood flow pump speed to minimize any effect of drainage collection canister’s (Figure 1) dead space dampening accurate measurements. This canister was 2 L, which is used to collect dialysis chamber drainage. Spent gas passes through the canister on the way to the ozone destruct device. (The canister acts as a trap for any drainage exiting the dialysis chamber, protecting the destruct device). At a flow rate of 0.9 L/min, the canister would be washed out 6.75 times over 15 minutes (13.5 L), virtually eliminating any dead space effect. The setup is pictured in Figure 1.
Treatment time was exactly 1 hour. Hence, 54 L of oxygen/ozone gas was available to the blood in the chamber for absorption.
Statistical analysis
Data analysis was used by Microsoft Excel.
Figure 1: Author Rowen receiving ozone dialysis treatment.
Note: Only the gray top of the Destruct device is visible behind the blood drip chamber that contains blood returning to the patient. Much brighter red indicates ozonated/oxygenated blood returning to the right arm.
RESULTS
A total of 85 measurements were made on 12 patients. Ozone output from the Zotzmann machine was analyzed throughout the treatment for accuracy and the machine showed virtually no variance over the hour, which created the charts below. Scatter charts were drawn using selected data and a linear trendline was generated.
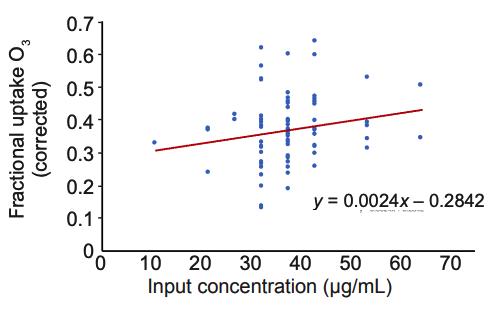
Figure 2 shows the fractional ozone uptake at each ozone μg/mL setting on the generator, after correction for the tubing/ equipment (no blood perfusion) ozone losses we measured. As shown in Figure 2, there was a significant variation in percentage uptake of ozone, with an overall average of about
Figure 2: Fractional ozone uptake vs. delivery concentration.
Based on these numbers, we noted that an “average” dialysis treatment would result in an average blood uptake of 37% of the ozone provided. For example, at 30 μg/mL, we would predict that about 600,000 μg of ozone is taken up for the onehour treatment. However, this needs to be adjusted by –23% to account for our measurements of the non-blood-loaded apparatus itself absorbing or degrading the ozone. That –23% correlated to –7 μg at a delivery concentration of 30 μg/mL. Therefore, 30 μg/mL – 7 μg/mL loss × 0.9 L/min × 60 minutes × 37% = ~460,000 μg blood uptake.
Ozone dialysis when delivered at 30 μg/mL appears to deliver an average of 3.28 times the amount of ozone compared to next highest method of delivery – OHT (10 passes) – which delivers 140,000 μg.
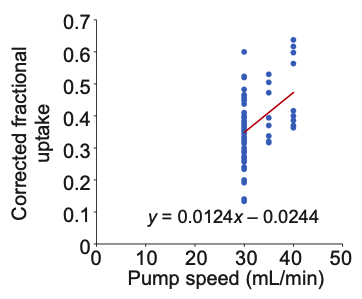
We then sought to determine the effect of pump speed on ozone absorption using data we collected at pump speeds of 30, 35, and 40 mL/min. Higher pump speeds (above 30 mL/min) were not commonly used because they required larger intravenous cannulas. While very likely giving superior ozone exposure to blood, we have concerns about the effects of larger catheters damaging our patients’ veins over repeated treatment insertions. The patients we measured were not recruited subjects for the analysis, but actual patients undergoing regular repeated sessions. Figure 3 shows fractional ozone uptake as blood flow speed through the dialysis chamber was increased. There was a trend toward more ozone absorption at a higher blood flow rate, as expected. Absorption appears to increase by up to 30% at 40 mL/min compared to 30 mL/min pump speed.
Figure 3: Fractional ozone uptake vs. blood flow rate.
DISCUSSION
Popular methods of ozone therapy deliver the following quantities of molecular ozone:
- Direct intravenous ozone gas at 20 mL of intravenous gas if provided at 40 μg/mL nets 800 μg of ozone injected.
- MAH (standard gravity method) using 200 mL of gas at 30–70 μg/mL into 200 mL of blood delivers 6000–14,000 μg of ozone (which could be affected by plastic collection bag absorption).
- Hyperbaric ozone single pass (generally 200 mL blood with 200 mL ozone gas at 70 μg/mL) delivers 14,000 μg ozone.
- High-dose hyperbaric ozone (10 passes of Lahodny’s method) delivers 140,000 μg of ozone.
- Ozone dialysis at a concentration of 30 μg/mL and a blood flow of 30 mL/min can easily deliver 3.28 or more times (460,000 μg) the amount of ozone delivered by the 10-pass method. At a higher blood flow (Figure 3) where the average uptake increased to 45%, the delivery may reach 1,242,000 μg × 45% = 558,900 μg with average treatment ozone concentration of 30 μg/mL. (1,242,000 μg reflects the 30 μg/mL ozone output minus 7 μg/mL apparatus loss). This correlates to a 4-fold higher delivery than the 140,000 μg of 10 pass.
- With use of 18-gauge catheters, and much higher blood flow, we would expect delivery to be significantly higher.
We have performed at least 400 sessions of ozone dialysis with no untoward effects observed. Patients who had received both dialysis and 10 pass treatments have shown near universal preference for dialysis delivery. Transient quicklypassing chest pressure is a not-infrequent reported effect of DIV delivery1 and an uncommon observation in 10 pass.¹⁴
Extracorporeal EBOO or ozone dialysis has been performed for at least two decades with no published reports of injuries. In discussions at ozone conferences, it is said to be highly effective. We see here that ozone dialysis is delivering, on average, at least 3 times more ozone in μg than other high dose delivery methods. Gravity MAH can treat 200 mL of blood with 200 mL of gas. At a commonly provided concentration of 50 μg/mL, 10,000 μg is delivered in total. If 70 μg/mL is used, 14,000 μg would be delivered. The dialysis treatment described is delivering some 32–46-fold more than gravity MAH.
Ozone dialysis clearly differs from other forms of blood ozone delivery. In DIV administration, ozone is injected as a slow bolus of tiny bubbles (27-gauge butterfly) directly into the vein.1 In conventional MAH, an aliquot of blood is exposed to ozone gas and reinfused into the patient under gravity. In hyperbaric ozone therapy, blood is also exposed to ozone gas but under pressure. Ozone dialysis permits passive diffusion of ozone into blood flowing through a dialysis chamber with facilitation of the diffusion gradient by countercurrent flow of ozone through the dialysis membrane. It may be considered a “kinder and gentler” delivery of ozone yet can possibly deliver over 400% more ozone by weight than its closest competitor – 10 passes.
Bocci²³ found that ozone therapy can induce hemolysis at concentrations above 75 μg/mL. Ozone dialysis employs an exceptionally safe concentration at less than half this threshold. Our findings mirror the biochemical findings of Di Paolo et al.¹⁹ mentioned above, whose team found a fourfold increase in thiobarbituric acid reactants and a proportionate reduction in blood reductants (thiols) after EBOO. In their study and the other early EBOO studies ozone was delivered at a very low concentration of 0.5–1.0 μg/mL but at a high blood flow rate: 4800 mL over 1 hour (80 mL/min for 60 minutes). Large catheters were employed (17-gauge).
In all our cases, we used 20-gauge intravenous catheters (Terumo SurFlow) for both the outflow and returning blood in contralateral antecubital or forearm veins, and which could achieve a flow rate up to 40 mL/min. The higher blood flow rate resulted in higher % absorption as expected (more surface area for uptake). However, we deliberately use 20-gauge intracaths for the routine therapy to protect patients’ veins. This was not a study of recruited subjects who might receive only a few needling sessions for experimental measurements. Our measurements were taken on patients receiving regular repeated therapy (vein needling) prescribed for their medical indication(s). Use of 18-gauge intracaths would permit a blood flow rate of 50 mL/min or greater, and nearly the entire body’s blood volume could be treated, but would be more “traumatic” to the veins over time. (In a single patient not included above, Rowen used 18-gauge catheters and achieved a maximum flow rate of 70 mL/min. Ozone uptake jumped significantly by more than 50% when flow rate reached 50 mL/min.)
Limitations
We were able to verify through both a dry dialysis chamber and one loaded with normal saline (not blood) that an average of 23% of ozone is lost through either absorption or reaction of ozone by/with the components we use for our ozone dialysis in our clinic. However, we did not determine if any of the unabsorbed ozone was destroyed after exiting the dialysis chamber. We do not know whether other dialyzing chambers and materials will show a similar loss. In addition, it is not possible to exactly predict each person’s uptake of ozone or how much ozone they will absorb in a given treatment as we do not know the intrinsic variables that may affect this. We observed variations between patients and also in the same patient’s multiple treatments.
Conclusion
This is the first report on ozone dosage delivery into blood by continuous countercurrent flow (also known as ozone dialysis). This method delivers ozone passively to flowing blood and can achieve ozone delivery of more than 300% higher than the standard higher dose delivery method, the 10-pass. Use of larger intravenous catheters with higher blood flow rate appears to significantly further increase delivery (uptake) of ozone compared to 20-gauge catheters. By our analysis, ozone dialysis (EBOO or RHP) is a remarkable advance in the administration of blood ozone therapy. Further studies on ozone dialysis delivery/uptake and efficacy are encouraged to determine best combination of catheter size (flow rate) vs. ozone concentration for both efficacy and vein protection.
Author contributions
RJR designed the study and was overseer of measurements. SG and TBS did the measurements. SG performed the computer analysis and graphs. All authors approved the final manuscript for publication.
Conflicts of interest
None.
Open access statement
This is an open access journal, and articles are distributed under the terms of the Creative Commons AttributionNonCommercialShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
References
- Robins H. Direct Intranenous Gas Applications in Ozone Therapy. Ozone Without Borders Annual Meeting, Tamarindo, Costa Rica. April. 2018.
- Smith NL, Wilson AL, Gandhi J, Vatsia S, Khan SA. Ozone therapy: an overview of pharmacodynamics, current research, and clinical utility. Med Gas Res. 2017;7:212-219.
- Viebahn-Haensler R, León Fernández OS. Ozone in medicine. The low-dose ozone concept and its basic biochemical mechanisms of action in chronic inflammatory diseases. Int J Mol Sci. 2021;22:7890.
- Wu X, Li Z, Liu X, et al. Major ozonated autohemotherapy promotes the recovery of upper limb motor function in patients with acute cerebral infarction. Neural Regen Res. 2013;8:461-468.
- Lahodny J. High dose ozone therapy (OHT). World Conference on Ozone Therapy in Medicine. Ancona, Italy. 2017.
- König B, Lahodny J. Ozone high dose therapy (OHT) improves mitochondrial bioenergetics in peripheral blood mononuclear cells. Transl Med Commun. 2022;7:17.
- Díaz-Luis J, Menéndez-Cepero S, Macías-Abraham C, FariñasRodríguez L. Systemic ozone therapy by rectal insufflation for immunoglobulin A deficiency. MEDICC Rev. 2018;20:29.
- Calunga JL, Paz Y, Menéndez S, Martínez A, Hernández A. Rectal ozone therapy for patients with pulmonary emphysema. Rev Med Chil. 2011;139:439-447.
- Bocci VA, Zanardi I, Travagli V. Ozone acting on human blood yields a hormetic dose-response relationship. J Transl Med. 2011;9:66.
- Rowen RJ. Oxidation and “unconventional” approaches to infection. Reference Module in Biomedical Sciences. 2021:B978-970- 912-818731-818739.800182-818738.
- Rowen RJ. Ozone and oxidation therapies as a solution to the emerging crisis in infectious disease management: a review of current knowledge and experience. Med Gas Res. 2019;9:232-237.
- Rowen RJ. Ozone therapy as a primary and sole treatment for acute bacterial infection: case report. Med Gas Res. 2018;8:121-124.
- Rowen RJ. Ozone therapy in conjunction with oral antibiotics as a successful primary and sole treatment for chronic septic prosthetic joint: review and case report. Med Gas Res. 2018;8:67-71.
- Rowen R. Safety and science of ozone high dose therapy. American Academy of Ozonotherapy annual meeting, Dallas Texas, USA. 2017.
- Bocci V, Zanardi I, Travagli V, Di Paolo N. Oxygenation-ozonation of blood during extracorporeal circulation: in vitro efficiency of a new gas exchange device. Artif Organs. 2007;31:743-748. 1
- Di Paolo N, Bocci V, Garosi G, et al. Extracorporeal blood oxygenation and ozonation (EBOO) in man. preliminary report. Int J Artif Organs. 2000;23:131-141.
- Jovanovic P. Personal communication. November 2014.
- Lingam P. Personal communication. March 24, 2022.
- Di Paolo N, Bocci V, Salvo DP, et al. Extracorporeal blood oxygenation and ozonation (EBOO): a controlled trial in patients with peripheral artery disease. Int J Artif Organs. 2005;28:1039-1050.
- Di Paolo N, Gaggiotti E, Galli F. Extracorporeal blood oxygenation and ozonation: clinical and biological implications of ozone therapy. Redox Rep. 2005;10:121-130.
- Di Paolo N, Bocci V, Cappelletti F, Petrini G, Gaggiotti E. Necrotizing fasciitis successfully treated with extracorporeal blood oxygenation and ozonization (EBOO). Int J Artif Organs. 2002;25:1194- 1198.
- Jovanovic P. Demonstration of Recirculatory Hemoperfusion, Ozone without Borders Annual Meeting, Aruba 2018.
- Bocci V. What Happens in the Intracellular Environment after Blood Ozonization? In: Bocci V, ed. Oxygen-ozone therapy: a critical evaluation. Dordrecht: Springer Netherlands; 2002:121-170.
Date of submission: February 1, 2022
Date of decision: March 23, 2022
Date of acceptance: April 21, 2022
Date of web publication: September 28, 2022